Geometry and model
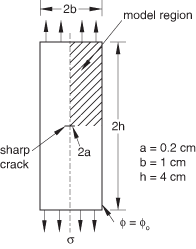
The problem geometry and boundary conditions are shown in Figure 1. The specimen is 10-mm thick, 20-mm wide, and 80-mm high, with a 4-mm crack at its center. The mesh near the crack is focused at the crack tip, with the element size growing as the square of the distance to the crack tip (with the first bounding node set representing the crack tip). A very fine mesh (see Figure 2) is used to capture accurately the gradients of concentration and stress near the crack tip. Four combinations of stress and mass diffusion analyses are presented:
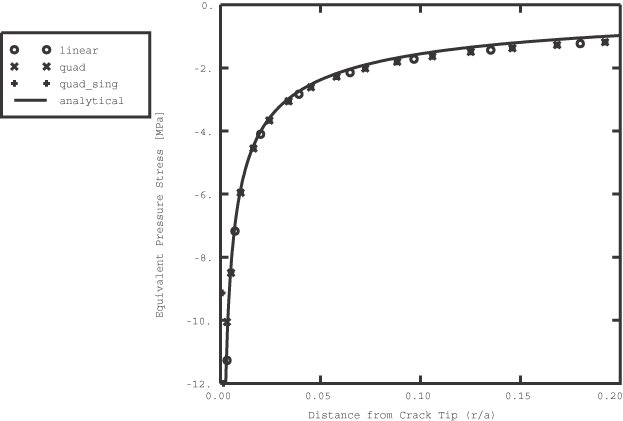
Stress analysis with quadratic elements and quarter-point spacing at the crack tip, followed by a mass diffusion analysis with linear elements.
Stress analysis with quadratic elements and quarter-point spacing at the crack tip, followed by a mass diffusion analysis with quadratic elements and quarter-point spacing at the crack tip.
Stress analysis with quadratic elements (no quarter-point spacing), followed by a mass diffusion analysis with quadratic elements (no quarter-point spacing).
Stress analysis with linear elements, followed by a mass diffusion analysis with linear elements.
The quarter-point spacing technique is used in fracture mechanics analyses to enforce a singularity at the crack tip, where r is the distance from the crack tip.
The sequentially coupled mass diffusion analysis consists of a static stress analysis, followed by a mass diffusion analysis. Equivalent pressure stresses from the static analysis are written to the results file as nodal averaged values. Subsequently, these pressures are read in during the course of the mass diffusion analysis to provide a driving force for mass diffusion.
The material properties for mass diffusion given by Fujii et al. (1982) are as follows.
Solubility:
Diffusivity:
where is the temperature in degrees Celsius and −273 is the absolute zero temperature.
Stress-assisted diffusion is specified by defining the pressure stress factor, as
where 8.31432 Jmol−1K−1 is the universal gas constant, 2.0 × 103mm3mol−1 is the partial molar volume of hydrogen in iron-based metals, and is the normalized concentration. The concentration dependence of is entered in Abaqus in tabulated form as shown in the input listings. It is important to note that although is defined in terms of normalized concentration, , the tabular data must be entered in terms of concentration,
The following properties are also used in the stress analysis: elastic modulus, 2.0 × 105Nmm−2, and Poisson's ratio, 0.3.
The specimen is maintained at a constant temperature of 325 K throughout the analysis. Under the initial steady-state conditions the specimen has a uniform concentration of 50 ppm, which corresponds to a normalized concentration of 265 N1/2mm−1. Normalized concentration is used as the primary solution variable (continuous over the discretized domain) and is given as the concentration divided by the solubility. The exterior of the specimen has a constant hydrogen concentration equal to the initial concentration. A 1 MPa distributed pressure is applied to the ends of the specimen, ramped linearly over the length of the step, and the steady-state distribution of hydrogen is obtained.