About the Lifecycle of a Study | ||||||
|
| |||||
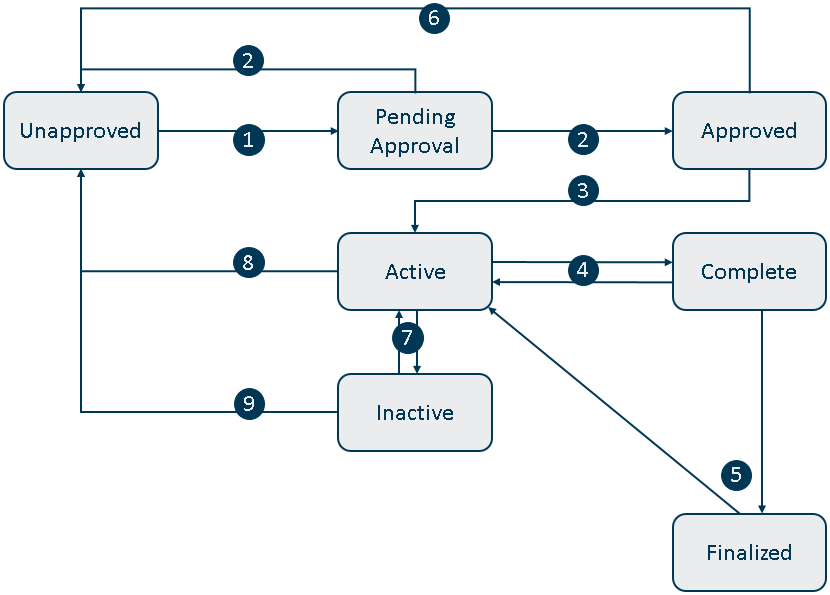
The lifecycle of a study has the following states and transitions:
 |
A study owner requests approval, including specifying one or more approver study members who have review rights. For more information, see Requesting Study Approval. |
 |
A specified approver member of the study reviews the study and approves it or rejects it for further edits. For more information, see Approving a Study. |
 |
A study owner activates the approved study. For more information, see Activating a Study. |
 |
A study owner completes the active study. For more information, see Completing a Study. If the completed study requires additional results later, a study owner who requested finalization or member with review rights can return it to the active state. |
 |
A study member with review rights finalizes the study. For more information, see Finalizing a Study. If the finalized study requires additional work, a study owner can return it to the active state. |
 |
A study owner rejects an approved study, returning it to the unapproved state. For more information, see Withdrawing a Study. |
 |
A study owner pauses an active study, returning it to the inactive state. If an inactive study is ready to be worked on again, a study owner can reactivate it. For more information, see Reactivating an Inactive Study. |
 |
If an active study requires a change in its definition, a study owner can reject it so that it returns to the unapproved state. For more information, see Updating an Active Study. |
 |
If an inactive study requires a change in its definition, a study owner can reject it so that it returns to the unapproved state. For more information, see Withdrawing a Study. |
Lifecycle States
- Unapproved: a study that you must fully define, review, and approve before
activation.
Collaborator and Owner study members can edit a study. Owners can request approval. Reader study members can view the study definition.
- Pending Approval: a defined study ready for review and signing.
No study members can make edits. The specified study members with review rights can approve or reject. The owner who requested approval can reject the study. Reader and Collaborator study members can view the study definition.
- Approved: a study whose design has been reviewed and signed off. But, which is not yet ready
for experiments to begin.
No study members can make edits. Owner study members can activate or reject. Reader and Collaborator study members can view the study definition.
- Active: a study that is ready for experiments to begin.
No study members can make edits. Owner study members can complete, reject, or pause. Reader and Collaborator study members can view the study definition.
- Complete: a study all of whose tasks are complete.
No study members can make edits. The specified study members with review rights can finalize or request further results. Reader study members can view the study definition.
- Finalized: a study whose tasks are complete and results approved.
No study members can make edits. Owner study members can reopen the study. Reader and Collaborator study members can view the study definition.
- Inactive: a paused study.
No study members can make edits. Owner study members can reactivate or reject for edits. Reader and Collaborator study members can view the study definition.