Introductory Concepts | ||||||
|
| |||||
Overview
Study definitions include data gathered from other resources. When you activate a study, it sends information to BIOVIA Foundation Hub and creates new resources. This topic describes how a study uses each resource.
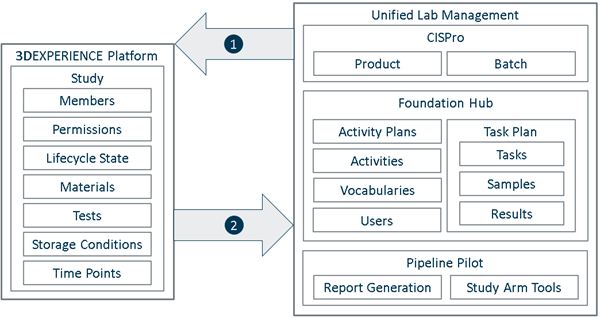
 |
Study Design and Tracking loads data from Unified Lab Management:
|
 |
Study Design and Tracking sends data to Unified Lab Management:
|
Study
A study defines the group of tests to perform on a material (target product) stored under specified conditions at the required time points. It also defines the members who can access the study according to their permissions. The lifecycle state of a study reflects its maturity and progress.
Stability studies are an integral part of the evaluation of a new drug or formulation. These provide information on the likely degradation products, helping to establish degradation pathways. Stability studies provide information on the intrinsic stability of the drug molecule. The results of stability studies are integral to the regulation processes.
Target
The study's target is the BIOVIA CISPro material (the drug molecule and its formulation) it investigates. Choose at least one lot of the material, with a sufficient amount of each lot to provide material for all the tests defined in the study.
For more information, see Products, Materials, Batches, and Lots and Amounts.
For stability investigations, study at least three lots of the material. The lots are manufactured in the minimum quantity of a pilot plant, using the same procedures as for production lots. The overall quality of the lots used in stability studies must be representative of the quality of production lots.
Test
A test defines the procedure to perform to investigate a material. Study Design and Tracking creates each test from a BIOVIA Foundation Hub activity. You can create multiple tests in a single step from an activity plan.
For more information, see Designing Tests.
Activities and Activity Plans
When you plan a study's tests, you can select the corresponding activities from those available in Foundation Hub. You can select individual activities for which to perform tasks. You can also select activity plans, each of which is a predefined group of associated activities, so that you can add multiple activities to your study in a single step. You can define activities and activity plans as favorites in either Study Design and Tracking or Foundation Hub. Items defined as favorites remain synchronized between the two apps for each user.
If you specify the GMP domain standard, you can only select approved activities for the study. BIOVIA Foundation Hub activities with draft, withdrawn, or superseded lifecycle states are not available.
Storage Conditions
The storage conditions define the location, temperature, humidity, orientation, and container for the material under investigation.
In stability studies, the storage conditions provide a stress test for the material under investigation. Stress tests can include the effect of temperatures (in 10°C increments), humidity where appropriate, oxidation, and photolysis. For example, the stress test could investigate the susceptibility of the drug molecule to hydrolysis; including a wide range of pH values, both in solution and in suspension.
For more information, see Defining Storage Conditions.
Time Points
The time points determine the schedule for performing the tests on the material.
For studies that aim to confirm expiry, time points are usually every 3 months for the first year, every 6 months for the second year, and annually thereafter. The overall minimum period of a long-term study is 12 months.
For accelerated stability studies, you usually require at least three time points, including the initial and final time points. If the results differ significantly between time points on this schedule, you can perform additional investigations. For example, you could investigate more time points or you could add more samples for the final time point. The overall minimum period for an accelerated study is 6 months.
Tasks and Task Plans
For each combination of test, storage conditions, and time point a task represents the work to carry out.
When you activate a study, this creates a Task Plan and Tasks in Foundation Hub and you can monitor their progress from Study Design and Tracking. The study's name populates the Task Plan's name and the Task Plan details include the study's identifier. The study's Summary tab reports the Task Plan's ID and provides a link to open the Task Plan in Foundation Hub.
Foundation Hub stores the Task Plan for a study in a Foundation Hub collaborative space. Each Foundation Hub user might have access to several collaborative spaces. When your study updates with information from the Task Plan it automatically connects to the collaborative space for the study.
For more information, see Defining Tasks and Reviewing a Study.
Samples
Each test creates a Sample in Foundation Hub for each storage condition of the CISPro lots of materials. All Tasks involved in the test at the specified time point use this Sample. This updates the total remaining amount of each lot in CISPro.
You can choose to use a "master" sample in your study. Specifying use of a master sample in a study results in storage of a single sample in each specified condition for each time point. For each sample, when a scientist performs a test they take aliquot from the master sample for the relevant time point and storage conditions. Non-destructive tests configured to reuse one sample for every time point are not included in the master sample.
For information on sample quantity data, see Amounts.
Vocabularies
The entries in the lists in the study creation and editing forms correspond to the vocabularies defined in Foundation Hub.
Results
Foundation Hub stores the results of the Tasks. The activity that defines the tests performed during the Task determines whether a result is acceptable. Only Tasks with acceptable results are completed and released in Foundation Hub, you can view tasks' statuses on the Monitoring tab of a study.
Users and Groups
For each study, you can import Foundation Hub groups as members of the study. Importing a group automatically adds the users in that group as members of the study. You can define the access role and review rights of each member individually. If a member of a group is already a member of the study, their addition is not processed and they retain their initial access role and review rights. Any different access roles or review rights assigned to the group do not apply to the existing study member.
If a member of an imported Foundation Hub group does not have a 3DPassport or they do not have the Study Manager role assigned, you cannot add them as a member of the study and a message provides information.